lonization is the process of removing an electron from an atom producing a free electron and positively charged ion. Due to collision of high-speed electron from atom, ionization takes place in triode tube. An ionization gauge is used for measurement of very low pressure of the order of 1 micron and below.
Construction of Ionization Gauge
The ionization gauge consists of A Triode Vacuum Tube. It has three terminals:
- A heated filament (cathode) to furnish electrons,
- A grid, and
- An anode plate.
These elements are housed in an envelope, which is connected to the vacuum system under test (where pressure is to be measured) as shown in figure 1. The grid is maintained at a positive potential of 100 – 350 V, while, the anode plate is maintained at a negative potential of about 2 – 50 V with respect to cathode. Grid acts as Electron collector and Anode acts as Positive Ion Collector.
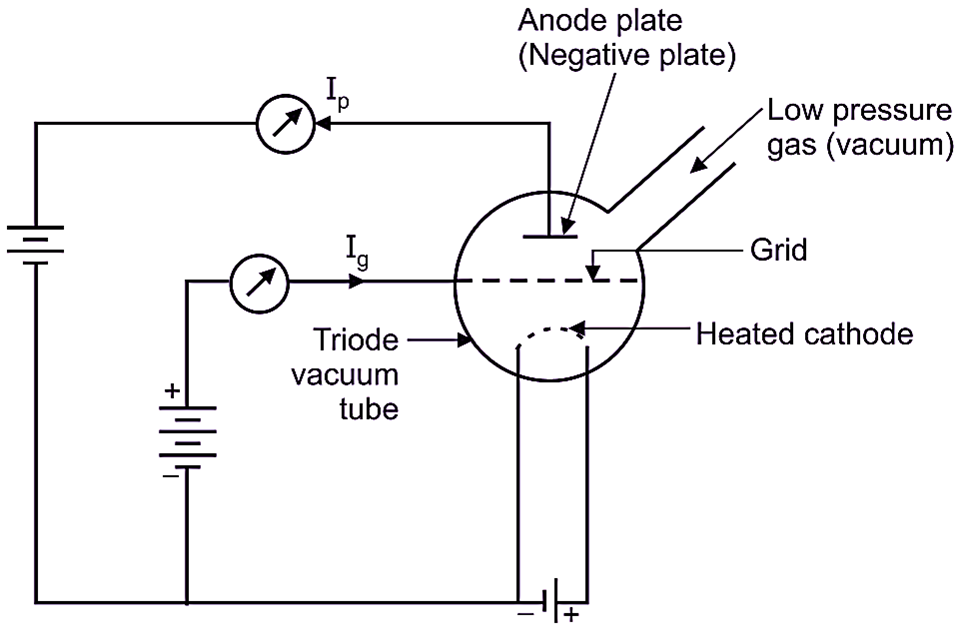
Fig. 1: Ionization Gauge
Working of Ionization Gauge
Let us consider that, pressure of gas below the value of atmospheric pressure (vacuum pressure) is to be measured. The Negatively charged electrons emitted by the heated cathode are attracted towards the positively charged grid. The electrons are accelerated due to the high positive charge present on the grid and therefore, electrons rapidly move towards grid (i.e. away from cathode). Some of the electrons are captured by the grid, producing grid current Ig. Electrons having high kinetic energy are not captured by grid and they are passed through the grid and collide with gas molecules, thereby causing ionization of gas atoms. The rate of ion production is proportional to the number of electrons available to ionize the gas and the amount of gas present. The process of knocking off an electron from an atom and thus producing a free electron and a positively charged ion is known as Ionization. The positive ions so produced are attracted towards anode plate (which is at negative potential) and anode plate current Ip, is produced in the plate circuit. The negative ions (electrons) so produced are collected by the high positive charge present on the grid. Thus, the grid current Ig produced is due to,
(a) The collected negative ¡ons on the grid and
(b) The captured electrons by the grid.
The ratio of the anode current Ip to the grid current Ig is a measure of the gas pressure ‘P’. The pressure of gas can be given as,
\[\text{P}=\frac{\text{1}}{\text{K}}\frac{{{\text{I}}_{\text{p}}}}{{{\text{I}}_{\text{g}}}}\]
Where, K = Sensitivity of gauge.
The value of K depends on triode tube geometry (shape), nature of gas and operating voltage.
Advantages of Ionization Gauge
- It is used for measurement of wide range of pressure (10-3 to 10-11 mm of Hg).
- Constant sensitivity for a given gas over wide range of measurement.
- Fast response to pressure changes.
- Possibility of process control and remote indication.
Disadvantages of Ionization Gauge
- High cost and complex electrical circuit.
- Its calibration varies with the gas.
- Decomposition of gas may take place by hot filament (cathode).
- The filament, if hot, can burn out quickly, if exposed to air.
- It is required to protect gauge by cut out in case of system leak or break.
- Contamination of gas, whose pressure is to be measured.
Applications of Ionization Gauge
- It can measure very low pressures, even negative gauge pressures.
- It is suitable to measure varying vacuum pressure, i.e., vacuum pressure of dynamic nature