Working Principle of Vacuum Distillation
Vacuum Distillation works on the principle of simple distillation with few changes. According to this method, the desired liquid is distilled at a temperature lower than its boiling point by the application of vacuum.
When vacuum is applied, it means that the pressure above the liquid surface is lowered which enhances the rate of distillation. Liquid begins to boil when its vapour pressure is equal to the pressure above the system i.e., liquid surface.
Large-Scale (Industrial Scale) Apparatus :
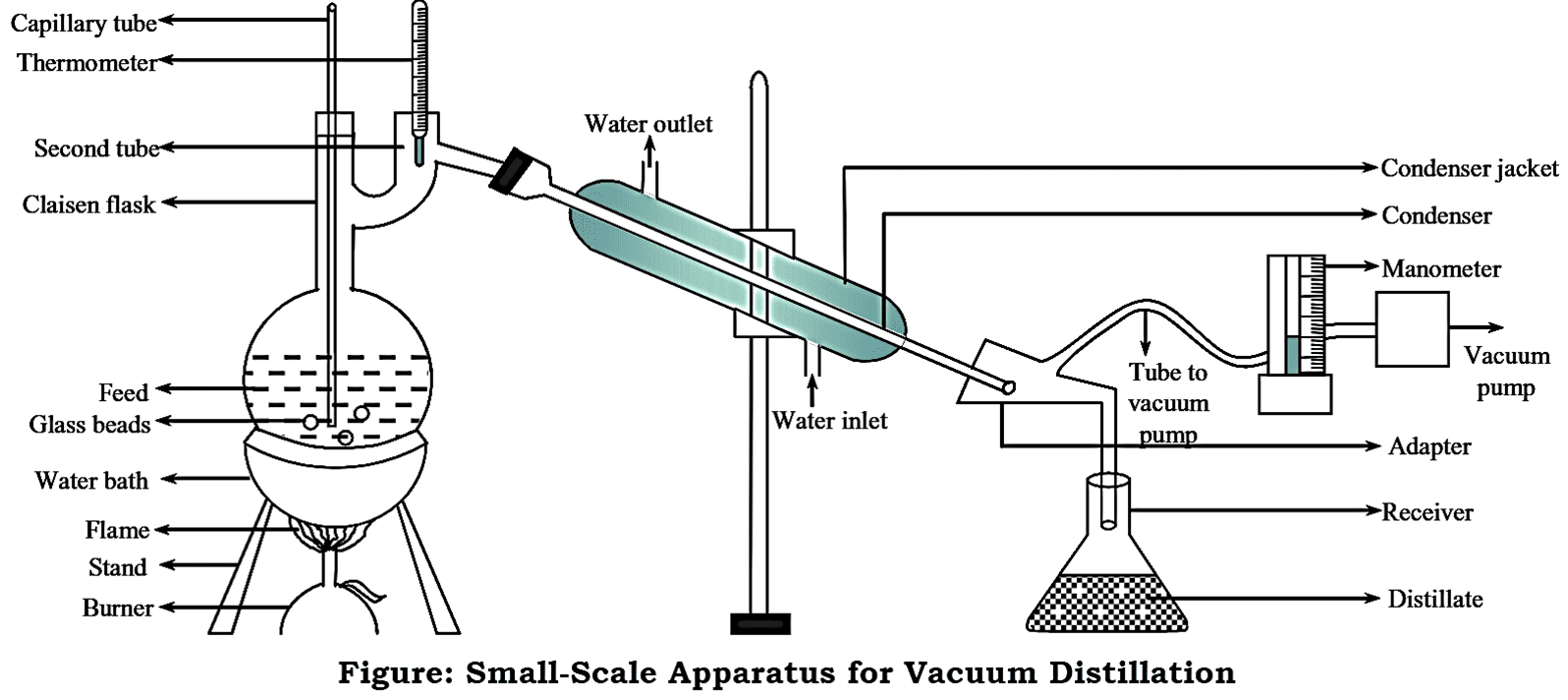
Construction of Vacuum Distillation Small-scale (laboratory scale)
The small-scale (laboratory scale) apparatus consists of a two-necked flask condenser (known as Claisen flask), receiver, adapter and a vacuum pump. The liquid feed is boiled using a water bath or oil bath. A capillary tube is placed in one of the necks of Claisen flask. This tube prevents bumping and splashing of feed liquid. The tip of the tube should be dipped into the feed, so as to draw out the stream of air bubbles from the flask. A thermometer is inserted in the second neck of the Claisen flask. Claisen flask is connected to a condenser which in turn is connected to a receiver via an adapter.
The adapter has a provision for vacuum pump. A manometer (pressure gauge) is placed between the vacuum pump and the receiver so as to monitor the pressure change.
Working of Vacuum Distillation Small-scale (laboratory scale)
The liquid feed to be distilled is introduced into the Clause flask. Few porcelain pieces (glass beads) are added to the liquid feed to avoid bumping and splashing. Bumping is also minimized by the capillary tube that is inserted into one of the necks of the flask. The required vacuum is applied. The liquid feed is then boiled using a water bath or oil bath. The liquid feed boils when its vapour pressure becomes equal to the pressure above the system under the influence of vacuum.
The mixed vapours enter the condenser and gets condensed. They are then collected in the receiver. The temperature at which the liquid begins to boil is noted from the thermometer. This temperature is found to be lower than the boiling point of the liquid.
Large-Scale (Industrial Scale) Apparatus :
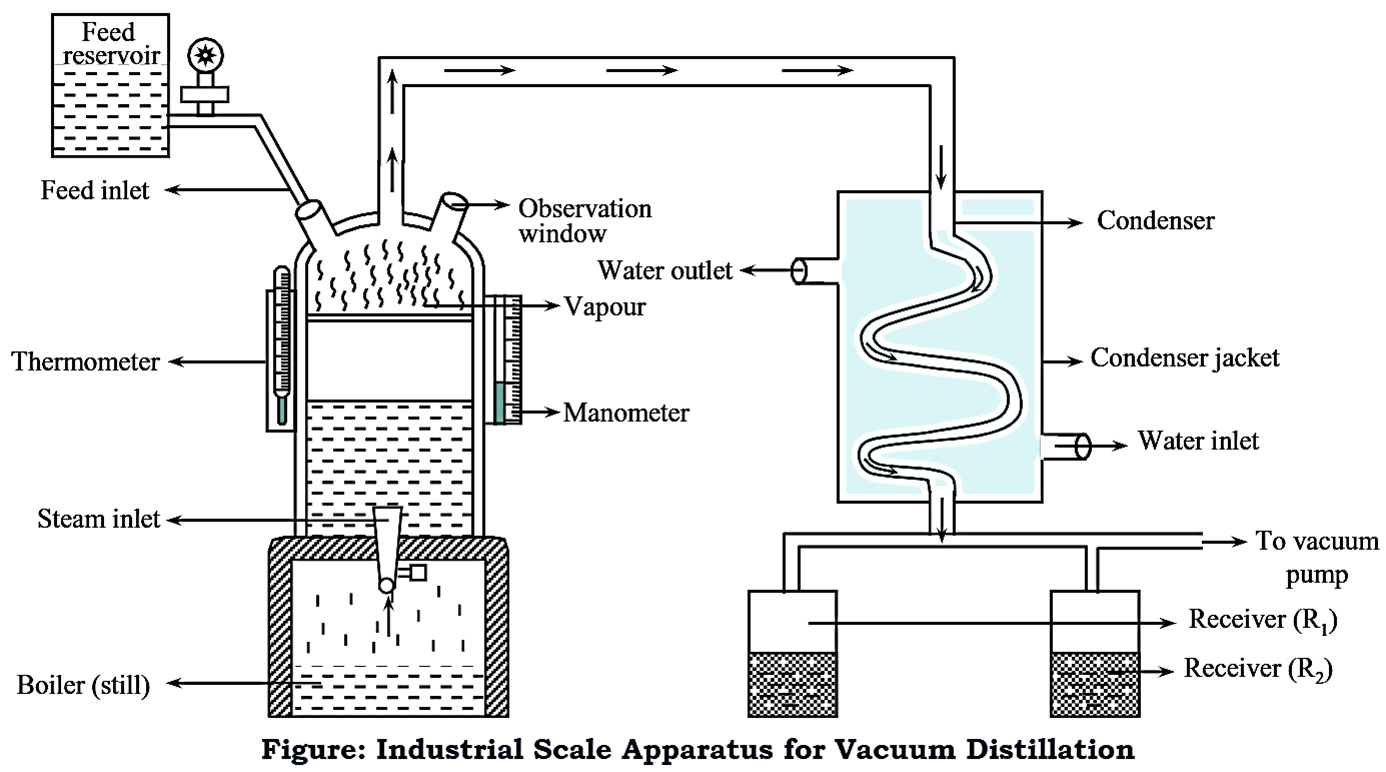
Construction of Vacuum Distillation Large-Scale (Industrial Scale) Apparatus
It is almost similar to apparatus used for simple distillation. The unit comprises the following essential parts,
1. Still
It consists of a large stainless steel or copper vessel, in which the liquid mixture to be distilled is placed. There is also a provision for thermometer. The hood of the still consists of an observation window to monitor the progress of the process. This observation window is also useful to see whether the liquid mixture to be distilled is at the right level or not. The hood also consists of a feed inlet. Steam is used as a heating medium which is circulated through the jacket. The base of the still is provided with a steam inlet and outlet.
2. Condenser
It is responsible for the condensation of vapours liberated from the still. It is made up of stainless steel and is covered with a jacket through which water is circulated in counter-current direction.
3. Receiver
Large scale distillation unit employs large metal containers made up of stainless steel. After condensation, the condensed vapours are carried into the receiver.
Working of Vacuum Distillation Large-Scale (Industrial Scale) Apparatus
The still is charged with the liquid mixture to be distilled, through a pipe from the reservoir for feed, at a controlled rate. Vacuum is applied by using a vacuum pump. Liquid mixture is heated with the aid of steam which is introduced into the jacket of the still. Under the influence of vacuum, the distillation temperature gets reduced and the liquid gets distilled at a temperature lower than its boiling point. The vapours are then fed to the condenser where they get condensed by the cool water circulating through the condenser jacket. The condensed vapours are then collected into the receiver.
Advantages of Vacuum Distillation
- Application of vacuum reduces the distillation temperature to a greater extent. Hence, distillation occurs at a faster rate. As the distillation temperature is low, thermolabile substances can be distilled without any deterioration or decomposition of active constituents.
Disadvantages of Vacuum Distillation
- Sudden application of vacuum to the hot boiling liquid may lead to foaming. However, excess foam formation can be avoided by the addition of anti- foaming agents such as capryl or octyl alcohol, silicones such as DC antifoam A etc.
- Violent splashing of the liquid feed, may cause entrainment of feed molecules in the condenser. This can be avoided by inserting a thin capillary tube in one of the neck of the Claisen flask.
- Vacuum distillation is not useful for the preparation of semi-solid or solid extract.
Applications of Vacuum Distillation
- Substances which are susceptible to degradation at higher temperatures are termed thermolabile (heat-sensitive). The active constituents in a drug may deteriorate and become inactive, when they are subjected to extraction and concentration at higher temperatures. Hence, these substances are distilled by vacuum distillation.
- The method can also be used to obtain granular and porous powders.