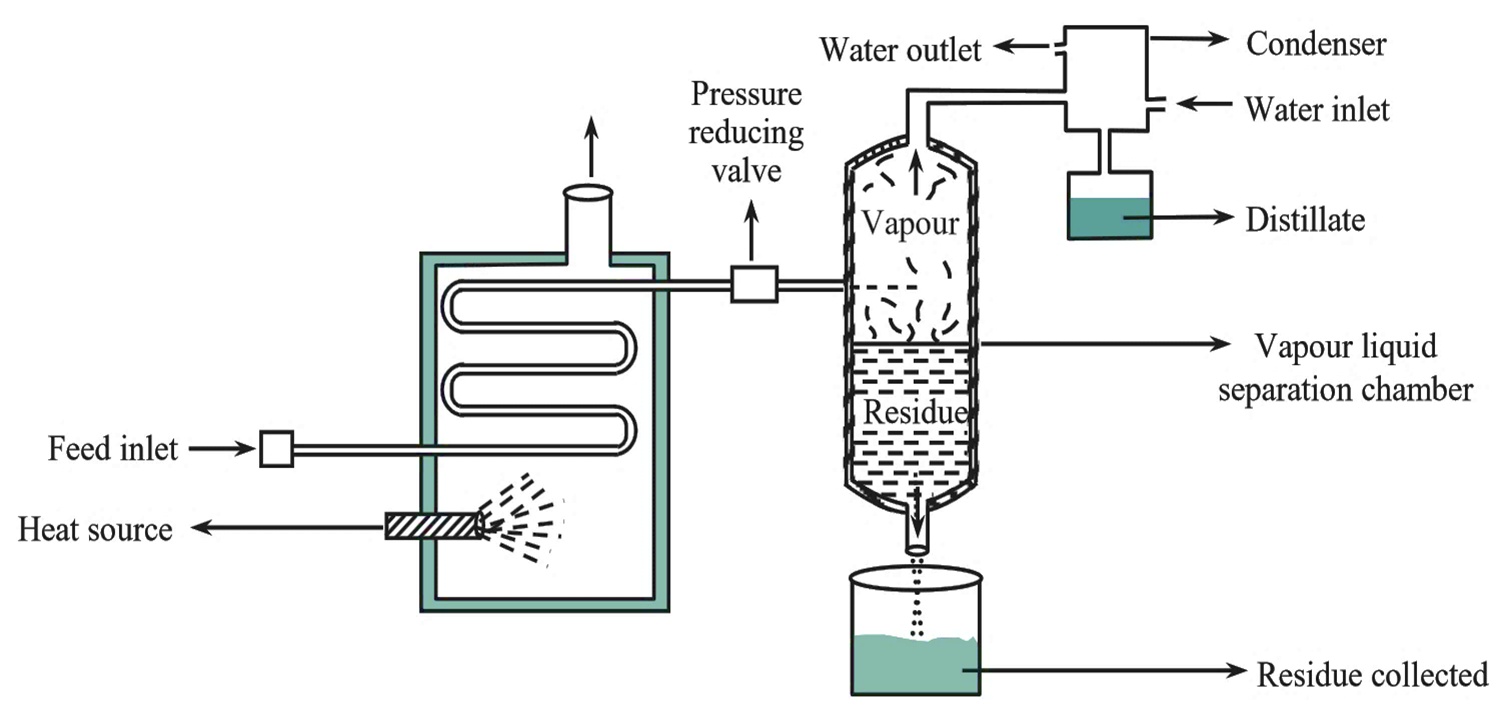
Figure 1: Flash Distillation Unit.
Working Principle of Flash Distillation
Flash distillation is also known as equilibrium distillation. The basic principle involved in this type of distillation is flash vapourization which involves sudden vapourization of hot liquid mixture to be separated by passing it from a high pressure area to a low pressure area. This results in sudden pressure drop which reduces the temperature of the chamber, which in turn results in sudden vapourization of the liquid mixture. Due to this, the component with high boiling point (less volatile component) gets condensed whereas the component with low boiling point (more volatile component) remains as vapor. The vapors and the liquid are kept in intimate contact with one another until equilibrium is achieved. Both the vapor and residual liquids are separated. Finally, vapors are subjected to condensation.
Construction of Flash Distillation
The Flash distillation apparatus consists of two chambers (see Figure 1).
Heating chamber
It is provided with a source of heat and also a provision for feed liquid. The feed reservoir is connected to a pump which forces the feed liquid into the heating chamber via a tube.
Vapour-Residual Liquid Separation Chamber
The terminal end of the heating chamber tube runs into the vapor-residual liquid separation chamber. A pressure-reducing valve is placed in the path of the tube which acts as a pressure regulator. Vapors (more volatile component) escape from the separation chamber through an outlet on top which is then directed towards condenser. The residual liquid conies out of the chamber through an outlet at the bottom.
Working of Flash Distillation
The feed liquid placed in the feed reservoir is pumped into the heating chamber (provided with a heating mechanism), at higher pressure. As a result, the temperature as well as the pressure inside the heating chamber gets raised. This liquid then enters into the vapor-liquid separation chamber via a pressure-reducing valve. Due to sudden drop in pressure, the hot liquid mixture undergoes partial vaporization or flashing. As a result, the temperature inside the separation chamber drops. The molecules of the low boiling component (more volatile component) remain as vapors while those of high boiling component (less volatile component) undergo condensation and form a residual liquid. Vapors and the condensed liquid are kept in intimate contact with one another. When equilibrium is attained both gets separated. The vapors escape out of the separation chamber through an outlet. These vapors are then directed towards the condenser where they are condensed and collected. The residual liquid is withdrawn from an outlet at the bottom of the chamber.
Advantages of Flash Distillation
- It is a useful method for separating pure components from multi-component systems such as crude oil which exhibits a narrow boiling range.
- It is a continuous process, however in order to maintain the equilibrium of the system. It is very important to note that the rate of feed (raw material) entering the system and the product leaving the system should be equal.
Disadvantages of Flash Distillation
- Components with similar volatilities cannot be separated.
- Flash distillation cannot be used for two-component systems, because it is not an efficient method to obtain entirely pure components.
Applications of Flash Distillation
- Flash distillation is used for the separation of multi-component liquids, where there is a wide difference in the boiling points of individual components. It is used for refining of crude oil in petroleum industry.
- It is widely used for desalination of sea water.