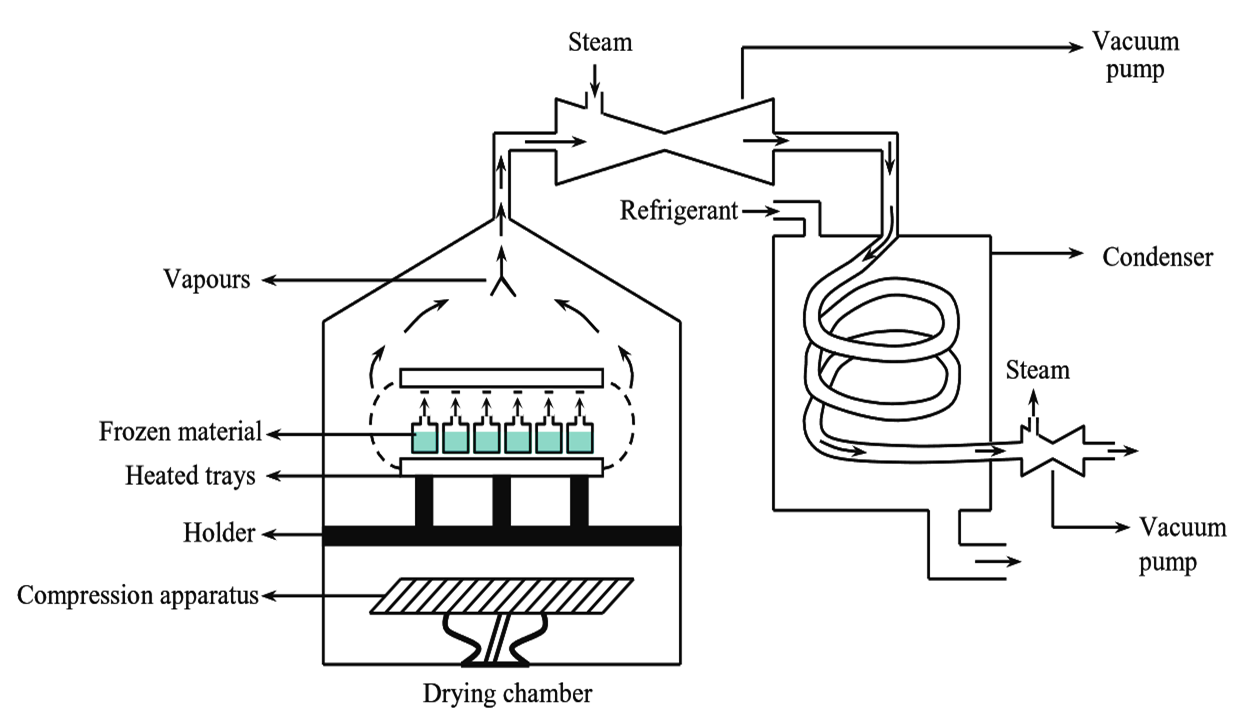
Figure 1: Freeze Dryer.
Working Principle of Freeze Dryer
Freeze drying is also called as lyophilization. It is the removal of water in the form of vapour directly from the frozen state (ice), without changing into the liquid state. Material is dried by exposing it to a temperature and pressure below its triple point. However, the temperature must be below the eutectic point during the process. Under these conditions the heat supplied acts as latent heat and sublimation occurs (i.e., conversion of solid into vapour). These formed water vapours are eliminated by passing it through a condenser, which is maintained at a temperature lower than the temperature of frozen material.
Construction of Freeze Dryer
The figure 1 shows the schematic representation of a typical industrial freeze dryer. It mainly consists of three parts.
- Drying Chamber
It is made of stainless steel which has a conical top and a flat bottom. Thermally heated trays are installed horizontally in the centre with the help of a holder. The compression apparatus is placed at the bottom (to carry out the mechanism of compression). A door is attached for the entry and removal of materials from the chamber. A vacuum pipe is connected to the chamber by means of an opening present at the conical top of the chamber.
- Vacuum Pump
It is present in between the drying chamber and the condenser, and is provided with an inlet for steam (steam jet).
- Condenser
Internally it consist of a coiled pipe surrounded by a mixture of acetone and dry ice (solid CO2), in order to maintain the temperature lower than the frozen material. Both the ends of the condenser are connected by the vacuum pump (along with the steam ejector). Distance between the drying chamber and the condenser must be in such a way that, it equals the mean free path travelled by the vapour molecules.
Working of Freeze Dryer
Initially, the solution is frozen in cold shelves (1-3) Kelvin/min with solutions) in a mixture of acetone and solid CO2. The quantity of the material to be dried (solution) should be optimum to facilitate complete freezing. In case the quantity is more, it can be evaporated by using vacuum tray dryers. While freezing, low conditions of temperature (- 50°C) and atmospheric pressure are maintained in the chamber.
Freezing is done at a high rate of 1-3 Kelvin/min facilitating the formation of large ice crystals with large holes. Preferably such crystals are of great importance in freeze drying, because they form a porous product on drying.
Frozen material spread on the heated trays is subjected to drying by maintaining the temperature and pressure lower than the triple point of water (when only water is present) i.e., 0.0098°C and 4.58 mm of Hg respectively. When liquids other than water are present, the temperature and pressure are maintained below the eutectic point. The temperature and pressure at which the solid sublimes to vapour is called as eutectic point (ranges from -10°C to 30°C).
Vacuum is applied (about 3 mm of Hg) to the frozen material and its temperature is raised to about 30°C within 2 hours. Now heat is applied at the rate of 2900 kJ/kg as latent heat due to which the frozen material sublimes into vapours.
Movement of ice layers is controlled by heat which regulates the formation of vapours above the frozen surface. It also prevents the melting of ice. The vapours formed are eliminated and the driving force is constituted by the difference in temperature of the drying chamber and the condenser. This is usually referred as primary drying in which the material loses about 98-99% of moisture. Remaining 1-2% of moisture is removed by increasing the temperature to about 50°C called as secondary drying which takes more than 10 hours of time.
Advantages of Freeze Dryer
- Most suitable method for highly thermolabile materials.
- The product obtained is porous, uniform and it is easy to recover.
- The proteinaceous constituents of materials (like tissues) are not denatured.
- Volatile materials can be dried as the loss of material is less.
- No hardening phenomena is observed.
- Oxygen sensitive materials can be dried.
- In case of vials and ampoules, the material is dried directly inside the containers, hence useful to maintain the sterility of products.
- Freeze dried products can be stored for long periods, because of least moisture content.
Disadvantages of Freeze Dryer
- Installation and operation costs are high.
- Time consuming process (requires more than 10 his).
- Product is prone to oxidation, if not packed under vacuum or inert gas.
- Not suitable for solutions containing non-aqueous solvents.
Applications of Freeze Dryer
- Freeze dryer is used for drying cultures of viruses and bacteria, living tissues, especially corneal and arterial tissues, plant extracts and antibiotics, enzymes, vitamins and steroid hormones.
- Food products like meat mushroom etc., including fruit juices and plant products (like coffee, tea) can be dried.
- It is the most commonly used equipment in the production of solutions, suspensions and injections.